Microscopic pores in brain cells may be a key to Parkinson’s
A toxic protein forms dynamic pores in the membranes of brain cells – and that may be the key to understanding how Parkinson’s disease develops. This is the conclusion of a new study from Aarhus University, where researchers have developed an advanced method to track molecular attacks in real time.

Parkinson’s disease often begins subtly. A slight tremor in the hand. A bit of stiffness. But over time, brain cells begin to die, and the symptoms worsen. The cause has long remained a mystery – but scientists may now be a step closer to an explanation.
At the center of attention is the protein α-synuclein, which plays a role in cell-to-cell communication in the healthy brain. In Parkinson’s, however, it starts to behave abnormally and clumps into toxic structures.
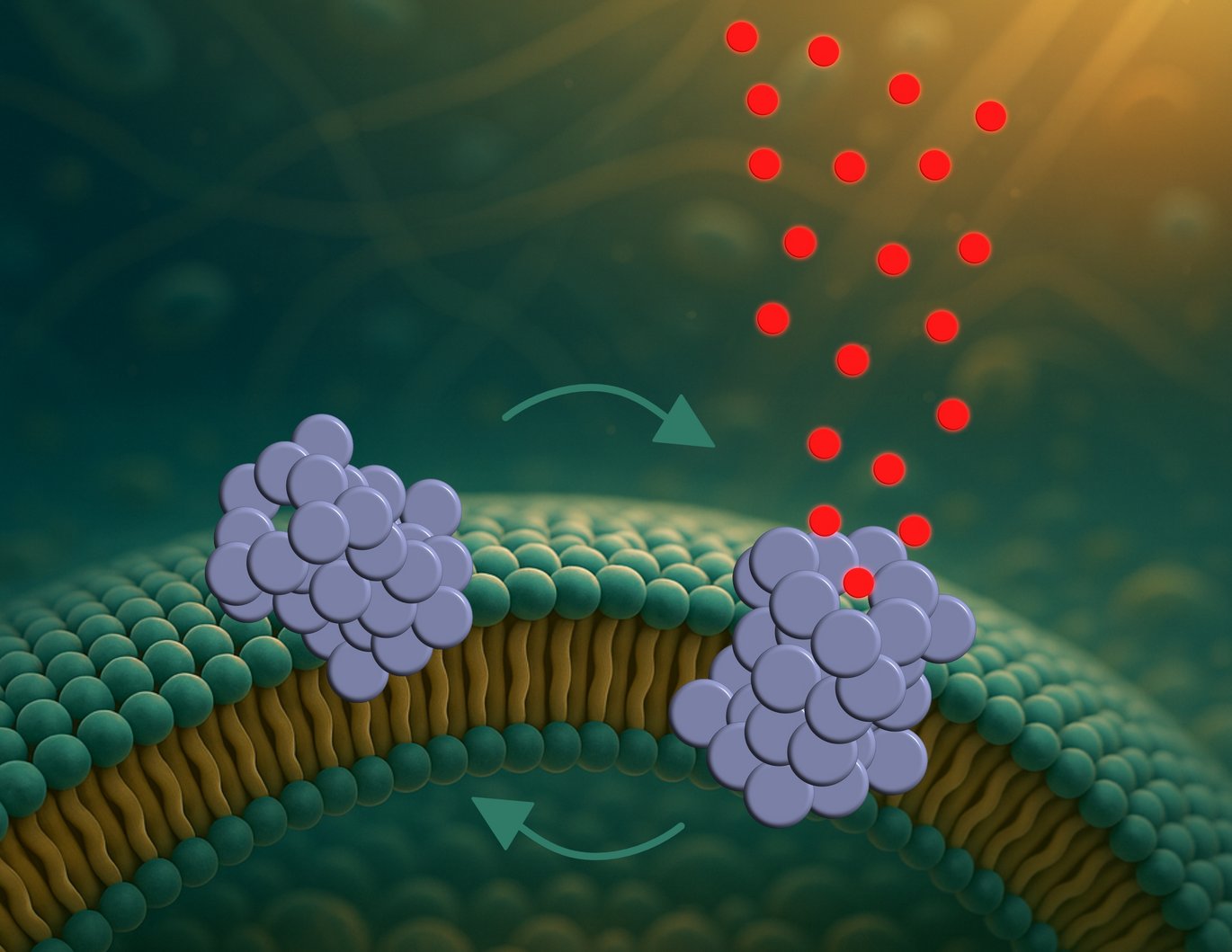
Until now, most research has focused on the large aggregates known as fibrils, which are visible in brain tissue from patients with Parkinson’s. But a new study focuses on smaller, less understood, and more toxic structures: α-synuclein oligomers. According to the researchers, these are the ones that drill microscopic holes in the membranes of nerve cells.
The study was recently published in the prestigious journal ACS Nano, published by the American Chemical Society.
Tiny revolving doors in the cells
“We are the first to directly observe how these oligomers form pores – and how the pores behave,” says Mette Galsgaard Malle, postdoctoral researcher at both Aarhus University and Harvard University.
The process unfolds in three steps. First, the oligomers attach to the membrane, especially at curved regions. Then they partially insert themselves into the membrane. Finally, they form a pore that allows molecules to pass through and potentially disrupt the cell’s internal balance.
But these are not static holes. The pores constantly open and close like tiny revolving doors.
“This dynamic behavior may help explain why the cells don’t die immediately,” says Bo Volf Brøchner, PhD student and first author of the study. “If the pores remained open, the cells would likely collapse very quickly. But because they open and close, the cell’s own pumps might be able to temporarily compensate.”
Molecular movie in slow motion
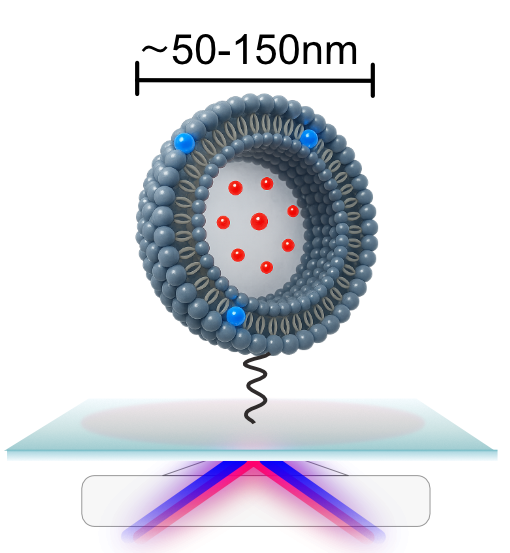
This is the first time such pore dynamics have been observed in real time. It was made possible by a newly developed single-vesicle analysis platform that allows researchers to follow interactions between individual proteins and individual vesicles.
Vesicles are small artificial bubbles that mimic cell membranes and serve as simplified models of real cells.
“It’s like watching a molecular movie in slow motion,” explains Mette Galsgaard Malle. “Not only can we see what happens – we can also test how different molecules affect the process. That makes the platform a valuable tool for drug screening.”
Long road to treatment
In fact, the team has already tested nanobodies – small antibody fragments – developed to specifically bind these oligomers. They show promise as highly selective diagnostic tools. However, as a treatment, there is still some way to go.
“The nanobodies did not block the pore formation,” says Bo Volf Brøchner. “But they may still help detect oligomers at very early stages of the disease. That’s crucial, since Parkinson’s is typically diagnosed only after significant neuronal damage has occurred.”
The study also shows that the pores are not formed randomly. They tend to emerge in specific membrane types – especially those resembling the membranes of mitochondria, the cell’s energy factories. This could indicate that the damage begins there.
One step at a time
However, the researchers emphasise that the study was conducted in model systems – not in living cells. The next step will be to replicate the findings in biological tissue, where more complex factors come into play.
“We created a clean experimental setup where we can measure one thing at a time. That’s the strength of this platform,” says Mette Galsgaard Malle. “But now we need to take the next step and investigate what happens in more complex biological systems.”
Additional information | |
| We strive to ensure that all our articles live up to the Danish universities' principles for good research communication(scroll down to find the English version on the web-site). Because of this the article will be supplemented with the following information: | |
| Funding | Lundbeck Foundation, Danish National Research Foundation |
| Read more | The scientific article in ACS nano Single-vesicle Tracking of α-Synuclein Oligomers Reveals Pore Formation by a Three-Stage Model |
| Contact | Mette Galsgaard Malle Postdoc, iNANO, Aarhus University and Wyss Institute, Harvard University Phone: +45 2849 1368 Email: malle@inano.au.dk Bo Volf Brøchner PhD student, iNANO–MBG, Aarhus University Phone: +45 5041 5596 Email: bovb@inano.au.dk |
